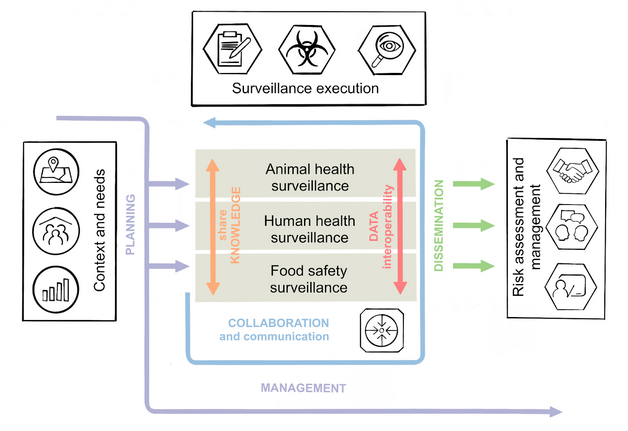
Introduction / Background¶
Zoonoses and antimicrobial resistance (AMR) continue to be significant impediments to human and animal health and to socioeconomic development worldwide [1], [2], [3]. National and international surveillance systems and monitoring programmes for zoonoses, zoonotic agents and AMR are means to combat these impediments. It is generally recognized that human and animal health are interconnected and that the transmission of zoonoses and AMR can essentially take place through various links of the human-animal interface e.g. the environment or food [4], [5]. This implies that surveillance cannot be addressed by the human or animal sector alone, but instead have to be a multisectoral and multidisciplinary collaboration [6]. This approach to collaboration is referred to as One Health. The benefits and importance of One Health Surveillance (OHS) are widely accepted, however, there are still gaps in surveillance or surveillance data that hinder a truly integrated OHS approach.
Within the EU, EFSA and ECDC have made substantial efforts to harmonize data collection and reporting within their sectors. These achievements are important assets for future OHS harmonization efforts, for example the Data Collection Framework (DCF [7]) and the SIGMA project (SIGMA [8]) from EFSA, as well as the European Surveillance System (TESSy [9]) from ECDC. Other ongoing joint efforts of these stakeholders support OHS data harmonization as well, for example the joint molecular typing database [10]. Another collaborative effort is the compilation of the yearly European Summary Reports (EUSRs) on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks and on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food (URL: https://www.efsa.europa.eu/en/publications). However, the current practice of provisioning OHS data in reports could be improved, specifically when it comes to national or research data not covered by European legislation. Moreover, no generic strategy for surveillance data reporting is currently available that could be adopted by all OHS related scientific disciplines including e.g. environmental science.
The One Health European Joint Programme (OHEJP)¶
In 2018, the One Health European Joint Programme (OHEJP) was established with the ambition to support the practical implementation of the One Health framework in Europe. The main focus of the new OHEJP is to reinforce collaboration between institutes by enhancing transdisciplinary cooperation and integration of activities by means of dedicated Joint Research Projects, Joint Integrative Project and through education and training in the fields of Foodborne Zoonoses (FBZ), Antimicrobial Resistance (AMR) and Emerging Threats (ET).
The One Health EJP Joint Integrative Project (JIP) “One health suRveillance Initiative on harmOnization of data collection and interpretatioN” (ORION) aims at establishing and strengthening inter-institutional collaboration and transdisciplinary knowledge transfer in the area of One Health Surveillance (OHS) data integration and interpretation. Detailed requirement analyses were performed within ORION to identify current best practices, resources and needs within the OHS community [11]. Results from the requirement analyses confirmed that cross-sectoral and multi-disciplinary communication, collaboration and knowledge exchange are still significant challenges for the European OHS community. In addition, the results reinforced the need for better harmonization of reports on OHS data, which could ultimately lead to improved mutual understanding and use of sector-specific data in future One Health analysis.
Another One Health EJP H2020 JIP currently in progress is called “MATRIX: Connecting dimensions in One-Health surveillance”, which aims to strengthen OHS practices and synergies across the entire surveillance pipeline between the public health (PH), animal health (AH) and food safety (FS) sectors within each country (expected end in December 2022). MATRIX builds upon the foundation laid out by ORION and other One Health EJP H2020 projects, while focusing on cross-sectoral hazards (i.e. Salmonella, Campylobacter, Listeria, and emerging threats, including antimicrobial resistance).
This OHS Codex/KIP therefore aims at establishing a high-level framework that supports collaboration, mutual understanding, knowledge exchange, data interoperability and coordination between OH sectors that will support integrated OHS data analyses, data interpretation and OH decission making. A manuscript describing the practical application scenarios of the OHS Codex/KIP has been also published [12].
Purpose¶
The purpose of this OHS Codex/KIP is to provide users with guidance and resources, e.g. tools, technical solutions, guidance documents, that support the design and planning of OH surveillance and monitoring activities as well as OH-driven data analysis and interpretation by the different OHS sectors. With that, the OHS Codex/KIP supports the ambitions outlined in chapter 3, 4, and 5 of the FAO, OIE and WHO document ‘Taking a Multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries” [13] (Tripartite Zoonoses Guide) by proposing specific resources that support putting a true One Health approach (as described in the Tripartite Zoonoses Guide) into practice in Europe.
Target community, main stakeholders and organizations¶
The primary target group for the OHS Codex/KIP are the official organisations and authorities involved in One Health. Other stakeholders, delivering data or in other ways are involved in One Health topics will have interest in knowing the system, the tools available and the lessons learned. According to the understanding of the authors elements of the OHS Codex/KIP will be useful for organizations or researchers that are involved in
- One Health surveillance implementation
- One Health surveillance data reporting
- One Health data harmonization and standardization
- Cross-sector risk management
Specifically, this includes
- the One HEalth EJP H2020 project consortium and their follow up organization
- national authorities in Europe involved in OHS
- European authorities and institutes, as e.g. EFSA, ECDC, EEA
- other stakeholders in OHS, as e.g. research organizations
Scope¶
The scope of the OHS Codex/KIP is to provide a framework to embrace different tools and methods that can enhance OHS data generation, analyses and interpretation. Currently, it is structured according to five core principles that were jointly defined by the OHS Codex/KIP curation board as critical for achieving this objective. The OHS Codex/KIP framework has the potential to be expanded by more principles in the future in case the scope / objective is broadened. In this case the OHS Codex/KIP could become a comprehensive hub of tools for OHS improvement.
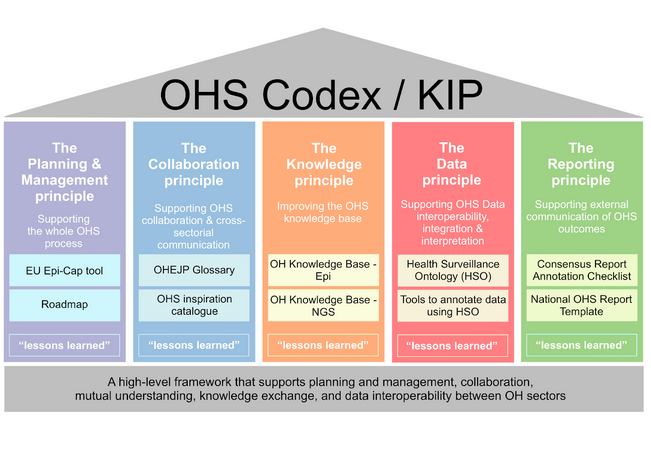
Fig. 2: The overview of the OHS Codex/KIP framework structured into five principles. The white boxes under each principle show examples of some of the solutions, tools and resources included into the OHS Codex/KIP. The “lessons learned” boxes describe practical One Health activities carried out, e.g.during the OH European Joint Programme (OHEJP) projects.
Principles¶
The OHS Codex/KIP framework is structured by five main principles. The OHS Codex/KIP describes each principle and within each of them it provides available solutions & methods to enhance OHS within each principle. These methods and tools were developed and tested within one of the EJP projects. However, the OHS Codex/KIP is designed as an updatable online resource that can be continuously expanded when new useful methods & solutions become available.
Download¶
The OHS Codex/KIP document is also available to download as:
References
| [1] | “Zoonotic Diseases: Progress Has Stalled.” European Food Safety Authority, 12 Dec. 2018, www.efsa.europa.eu/en/press/news/181212. |
| [2] | “Zoonoses.” World Health Organization, World Health Organization, 19 July 2017, www.who.int/topics/zoonoses/en/. |
| [3] | “Antimicrobial Resistance.” World Health Organization, World Health Organization, www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance. |
| [4] | A European One Health Action Plan against Antimicrobial Resistance |
| [5] | Taking a Multisectoral, One Health Approach:A Tripartite Guide to Addressing Zoonotic Diseases in Countries |
| [6] | Taking a Multisectoral, One Health Approach:A Tripartite Guide to Addressing Zoonotic Diseases in Countries |
| [7] | https://www.efsa.europa.eu/en/supporting/pub/en-444 |
| [8] | https://www.efsa.europa.eu/en/supporting/pub/en-1428 |
| [9] | https://ecdc.europa.eu/en/publications-data/european-surveillance-system-tessy |
| [10] | EFSA (European Food Safety Authority), 2014. Technical specifications for the pilot on the collection of data on molecular testing of food-borne pathogens from food, feed and animal samples. EFSA supporting publications 2014;11(12):EN‐712, 58 pp. doi: 10.2903/sp.efsa.2014.EN-712 |
| [11] | ORION. (2020, April 16). Deliverable-JIP1-D2.3 Report on requirement analysis for an “OH Knowledge Base – Integration” (ORION). Zenodo. http://doi.org/10.5281/zenodo.3754596 |
| [12] | Filter M., Buschhardt T., Dórea F., Lopez de Abechuco E., Günther T., Sundermann E. M., Gethmann J., Dups-Bergmann J., Lagesen K & Ellis-Iversen J. One Health Surveillance Codex: promoting the adoption of One Health solutions within and across European countries, One Health, Volume 12, 2021. https://doi.org/10.1016/j.onehlt.2021.100233. http://doi.org/10.5281/zenodo.3754596 |
| [13] | Taking a Multisectoral, One Health Approach:A Tripartite Guide to Addressing Zoonotic Diseases in Countries |